Tularemia Fact Sheet
|
Tularemia is also known as “rabbit fever” and “deerfly fever” and is caused by infection with the bacteria Francisella tularensis, found throughout North America, Europe, and Asia. There are two subspecies of the bacteria, Type A, found in North America, and Type B found in Europe. Type A is highly virulent, whereas Type B causes milder forms of the disease. In the United States, approximately 200 human cases are reported each year, with less than 2% being fatal. Most cases occur in rural areas of the south-central and midwestern states during the summer months. From 1990 to 2000, 56% of all reported tularemia cases in the U.S. were in four states, Arkansas, Missouri, South Dakota, and Oklahoma. Delivery: Tularemia is not transmissible from person-to-person, so infected humans do not need to be quarantined. The disease is acquired either by being bitten by infected ticks, deerflies, mosquitoes, or other insects that feed on infected animals, by handling infected animal carcasses, consuming contaminated food or water, inhaling the bacteria, or through the eyes. Animals that can be infected include rodents and rabbits. Production: Although the bacteria does not form spores, F. tularensis can survive for weeks at low temperatures in moist soil, water, hay, straw, and decaying plants and animals. The bacteria can be isolated from these sources and grown. F. tularensis is considered a potential bioterrorism agent because it can be aerosolized to enhance inhalation exposure and as few as 10-50 cells can cause disease. Tularemia is rarely lethal when treated with antibiotics. History: F. tularensis was initially identified in 1912 after ground squirrels in Tulare County, California, were observed to have a plague-like illness. Waterborne outbreaks occurred in Europe and the Soviet Union in the 1930's and 40's. In 1939, tularemia cases in the United States peaked at 2,291. In the past, Japan, the Soviet Union, and the United States have worked to weaponize F. tularensis, with the Soviet Union continuing its work into the 1990s. |
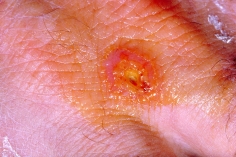
Effects: Tularemia symptoms usually appear within 3-5 days of infection, but can appear up to 2-3 weeks after infection. Initial symptoms include fever, chills, headaches, diarrhea, muscle aches, joint pain, dry cough, sore throat, and progressive weakness. Prolonged infection can result in pneumonia, chest pain, bloody sputum, difficult breathing, and death. Depending on the route of infection, skin or mouth ulcers, swollen and painful lymph glands, and swollen and painful eyes can occur as well. If aerosolized tularemia is used in a bioterrorist attack, the majority of symptoms would involve severe respiratory illness, including pneumonia and systemic infection. Treatment: Preliminary testing for tularemia infection can be done in two hours; however, confirmation can take another 24 to 48 hours. Antibiotic treatment is generally started before confirmation. Several different antibiotics can be used, including streptomycin, gentamicin, doxycycline, and ciprofloxacin. Before antibiotic treatments were available, pulmonary and blood poisoning cases of tularemia had a mortality rate of 30-60%. A tularemia vaccine produced by United States Army Medical Research Institute for Infectious Diseases has been given to laboratory workers and others at high-risk for infection. That vaccine is classified as an investigational new drug and is not licensed by the Food and Drug Administration. No vaccines are currently available for public use. |
|||
(Sources: Centers for Disease Control and Prevention, National Institute of Allergy and Infectious Diseases, University of Pittsburgh Medical Center Center for Biosecurity) |
||||